In continuum mechanics, an equation of state suitable for modeling solids is naturally rather different from the ideal gas law. A solid has a certain equilibrium volume V0, and the energy increases quadratically as volume is increased or decreased a small amount from that value. The simplest plausible dependence of energy on volume would be a harmonic solid, with
The next simplest reasonable model would be with a constant bulk modulus
Murnaghan equation of state
A more sophisticated equation of state was derived by Francis D. Murnaghan of Johns Hopkins University in 1944. To begin with, we consider the pressure
and the bulk modulus
Experimentally, the bulk modulus pressure derivative
is found to change little with pressure. If we take B' = B'0 to be a constant, then
- B = B0 + B'0P(4)
where B0 is the value of B when P = 0. We may equate this with (2) and rearrange as
Integrating this results in
or equivalently
Substituting (6) into 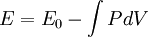 then results in the equation of state
for energy.
then results in the equation of state
for energy.
Many substances have a fairly constant B'0 of about 3.5.
Birch–Murnaghan equation of state
The third-order Birch–Murnaghan isothermal equation of state, published in 1947 by Francis Birch of Harvard, is given by:
Again, E(V) is found by integration of the pressure:
References
- ^ F.D. Murnaghan, 'The Compressibility of Media under Extreme Pressures', in Proceedings of the National Academy of Sciences, vol. 30, pp. 244-247, 1944.
- ^ Francis Birch, 'Finite Elastic Strain of Cubic Crystals', in Physical Review, vol. 71, pp. 809-824 (1947).
https://www.chemeurope.com/en/encyclopedia/Birch%E2%80%93Murnaghan_equation_of_state.html

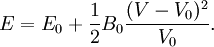
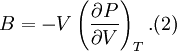
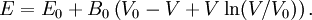
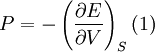
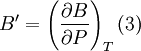
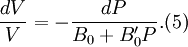
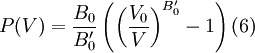
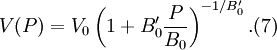
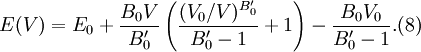
![P(V)=\frac{3B_0}{2} \left[\left(\frac{V_0}{V}\right)^\frac{7}{3} - \left(\frac{V_0}{V}\right)^\frac{5}{3}\right] \left\{1+\frac{3}{4}\left(B_0^\prime-4\right) \left[\left(\frac{V_0}{V}\right)^\frac{2}{3} - 1\right]\right\}](https://www.chemeurope.com/en/encyclopedia/images/math/e/a/c/eac6109009a60ca850d784ae7b8783dd.png)
![E(V) = E_0 + \frac{9V_0B_0}{16} \left\{ \left[\left(\frac{V_0}{V}\right)^\frac{2}{3}-1\right]^3B_0^\prime + \left[\left(\frac{V_0}{V}\right)^\frac{2}{3}-1\right]^2 \left[6-4\left(\frac{V_0}{V}\right)^\frac{2}{3}\right]\right\}](https://www.chemeurope.com/en/encyclopedia/images/math/9/c/6/9c68b3fd8a2ccf34b99e89fb629b6391.png)
Great Article. Do visit our website https://www.mastersindia.co/gst-calculator/
ReplyDelete