The bond energy between the charges is proportional to q1 * q2/r
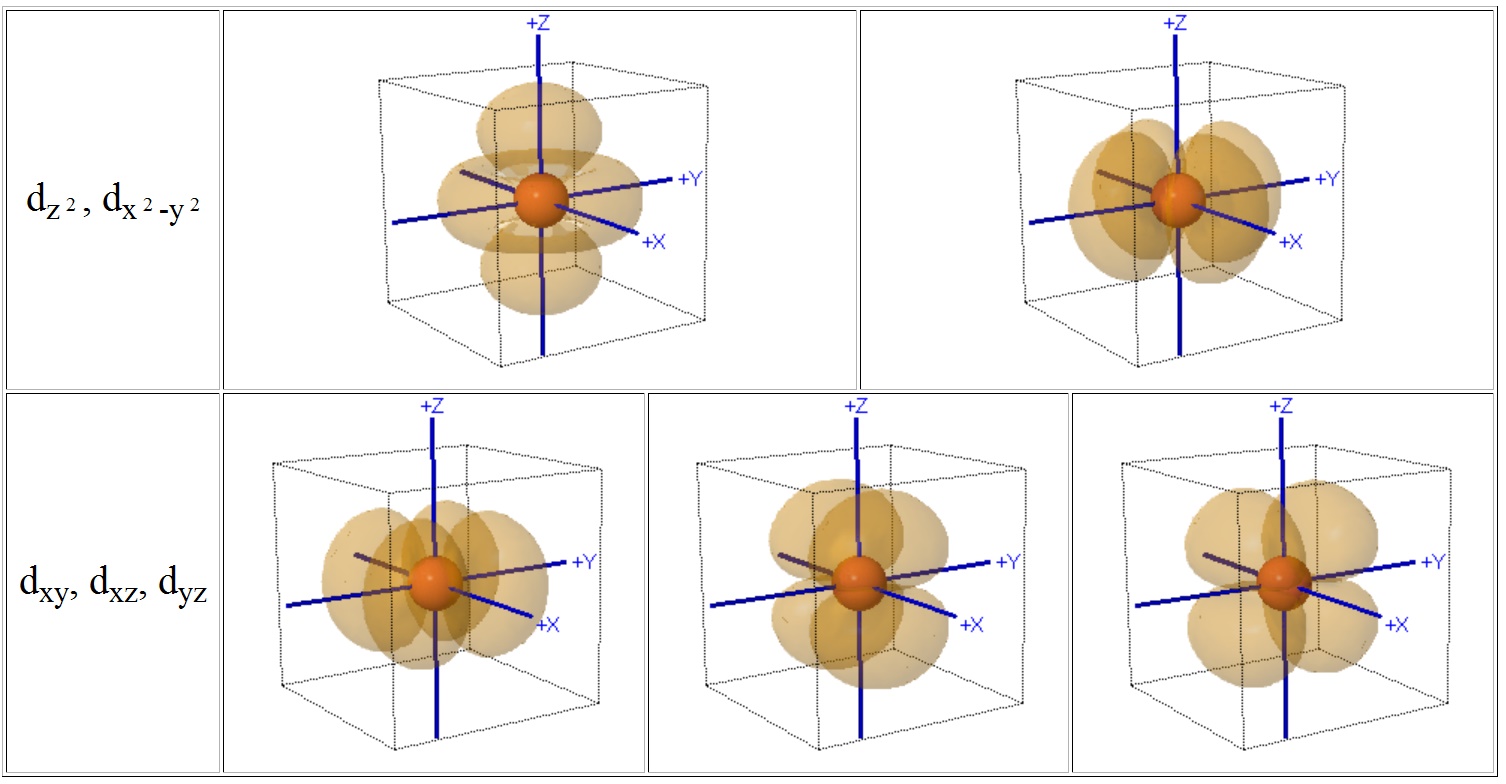
For transition metal cations that contain varying numbers of d electrons in orbitals that are NOT spherically symmetric, however, the situation is quite different. The shape and occupation of these d-orbitals then becomes important in an accurate description of the bond energy and properties of the transition metal compound.
To be able to understand and use CFT then, it is essential to have a clear picture of the shapes (angular dependence functions) of the d-orbitals.
To continue click on the link below:
http://wwwchem.uwimona.edu.jm/courses/CFT.html

No comments:
Post a Comment